New Findings!
 Alzheimer’s disease (AD) currently affects nearly 1.5 million people in the US and this number is expected to quadruple over the next 20 years, making it a major public health risk1. Although abnormal metabolism of the amyloid precursor protein resulting in excess accumulation of toxic amyloid-β (Aβ) peptides is believed to be the principal cause of AD2; 3; 4, the disease continues to be difficult to diagnose and there are currently no effective treatments for this devastating illness. Improved diagnostic methods and possible new treatments are therefore critically needed.
Alzheimer’s disease (AD) currently affects nearly 1.5 million people in the US and this number is expected to quadruple over the next 20 years, making it a major public health risk1. Although abnormal metabolism of the amyloid precursor protein resulting in excess accumulation of toxic amyloid-β (Aβ) peptides is believed to be the principal cause of AD2; 3; 4, the disease continues to be difficult to diagnose and there are currently no effective treatments for this devastating illness. Improved diagnostic methods and possible new treatments are therefore critically needed.
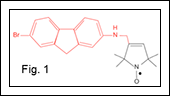
To address this need, we have developed a small molecule with unique properties that may both improve capability to diagnose AD as well as potentially treat the disease. This compound, which we have named spin-labeled fluorene (SLF) is unique in that one part of the molecule (red in Fig. 1) binds to Aβ making it an ideal candidate for AD therapeutic target engagement5. The innovative aspect of this compound, however, is the addition of a nitroxide spin label (black, in Fig. 1). The bifunctional character of SLF can be applied in two ways: (i) therapeutically, via the fluorene altering Aβ structure and the spin-label lowering oxidative stress in the vicinity of Aβ, and (ii) as a diagnostic agent by targeting the paramagnetic N-O species to assemblies of Aβ.
Preliminary Data
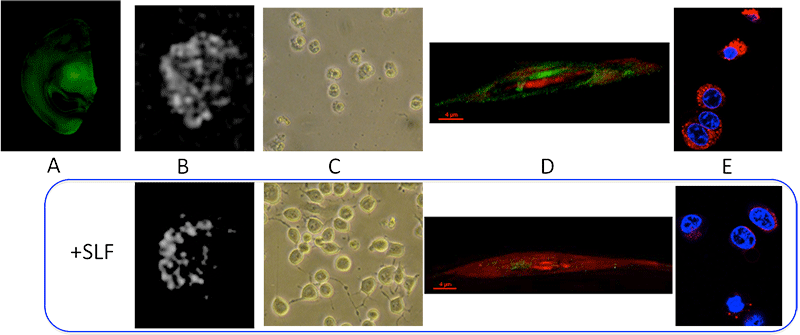
Multi-functional activity of the SLF. The green fluorescence panel (A) shows the Alzheimer's Aβ peptide detected in a single hemisphere of a coronal slice from a brain of an Alzheimer's model mouse. The other panels compare results when the SLF compound absent (top row) or present (bottom row) in the experiment. In (B) SLF provides contrast in regions of the brain slice high in Aβ. Shown is the T2* weighted MRI image of comparable mouse brain slices. The loss in MRI intensity is evident in the SLF-treated slice. Experiment (C) shows how the SLF provides protection against Aβ toxicity in cultured neurons. Addition of Aβ to N2A cells results in the loss of cell viability after 48 hours (top), whereas inclusion of 10 micromolar SLF completely blocks Aβ toxicity (bottom (C, bottom panel). Finally, we can also image the ability of the SLF to reduce oxidative stress in neuronal culture that is challenged with elevated levels of the Aβ peptide. This is demonstrated in (E), where a fluorescent indicator for reactive oxygen species (red color) is nearly absent in cells that have been treated with the SLF compound.
Citations:
- Brookmeyer, R., Gray, S. & Kawas, C. (1998). Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health 88, 1337-42.
- Hardy, J. (1992). An 'anatomical cascade hypothesis' for Alzheimer's disease. Trends Neurosci 15, 200-1.
- Hardy, J. A. & Higgins, G. A. (1992). Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184-5.
- Hardy, J. & Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353-6.
- Sperling, R. A., Jack, C. R., Jr. & Aisen, P. S. (2011). Testing the right target and right drug at the right stage. Sci Transl Med 3, 111cm33.
Other New Findings ››